U.S. Department of Health and Human Services
Federal Authority for Medicaid Special Needs Plans and Their Relationship to State Medicaid Programs
Paul Saucier, Jessica Kasten and Brian Burwell
Thomson Reuters
January 2009
PDF Version: http://aspe.hhs.gov/daltcp/reports/2009/leghist.pdf (14 PDF pages)
This issue brief was prepared under contract between the U.S. Department of Health and Human Services, Office of the Assistant Secretary for Planning and Evaluation, Office of Disability, Aging and Long-Term Care Policy (DALTCP) and Thomson Health Care. For additional information about this subject, you can visit the DALTCP home page at http://aspe.hhs.gov/_/office_specific/daltcp.cfm or contact the ASPE Project Officer, Hunter McKay, at HHS/ASPE/DALTCP, Room 424E, H.H. Humphrey Building, 200 Independence Avenue, S.W., Washington, D.C. 20201, Hunter.McKay@hhs.gov.
Overview and Purpose
This brief, the first in a series of three, reviews the history and current status of federal Medicare Special Needs Plan (SNP) authority, with particular attention to provisions of interest to state Medicaid programs. Medicare SNPs were first authorized in December 2003, in the Medicare Prescription Drug, Improvement, and Modernization Act (MMA) of 2003, the same legislation that created the Medicare Part D prescription drug program.1 A SNP is a type of Medicare Advantage plan that may restrict enrollment to specified groups of Medicare beneficiaries believed to benefit from specialty care tailored to their group characteristics. In response to concerns that many SNPs have not, in fact, been offering specialty models of care, federal legal authority has been amended twice recently, first in December 2007 as part of the Medicare, Medicaid, and SCHIP Extension Act (MMSEA) of 2007, and again by the Medicare Improvements for Patients and Providers Act (MIPPA) of 2008 in July 2008.2
The Congressional actions of December 2007 and July 2008, coming less than a year apart, resulted in a one-year freeze in the program, but also extended the programs authority from the original sunset date of December 31, 2008 to the current end date of December 31, 2010. When the Centers for Medicare and Medicaid Services (CMS) resumes accepting applications in 2009 for contract year 2010, new requirements will be in place, but unless federal authority is further extended, the revised program will have only one year to run. Figure 1 provides major points in the evolution of SNP authority.
This brief focuses on SNP provisions impacting state Medicaid programs that have or are considering entering into contracts with SNPs to integrate or coordinate Medicaid long-term care services with Medicare primary, acute and prescription drug services for dually eligible beneficiaries. SNPs have been promoted as a mainstream vehicle for integrating Medicare and Medicaid services and are being employed to that end in a few notable states. To date, many SNPs have had no formal relationships with state Medicaid programs. In the future, SNPs proposing to serve dually eligible beneficiaries will be required to have contracts with state Medicaid programs. This and other key changes to the program are outlined here. Subsequent briefs will describe the types of relationships that SNPs had with state Medicaid programs as of 2008, and report on best practices in SNP-state contracting.
| FIGURE 1. Medicare Special Needs Plan (SNP) Authority Over Time(as of December 2008) |
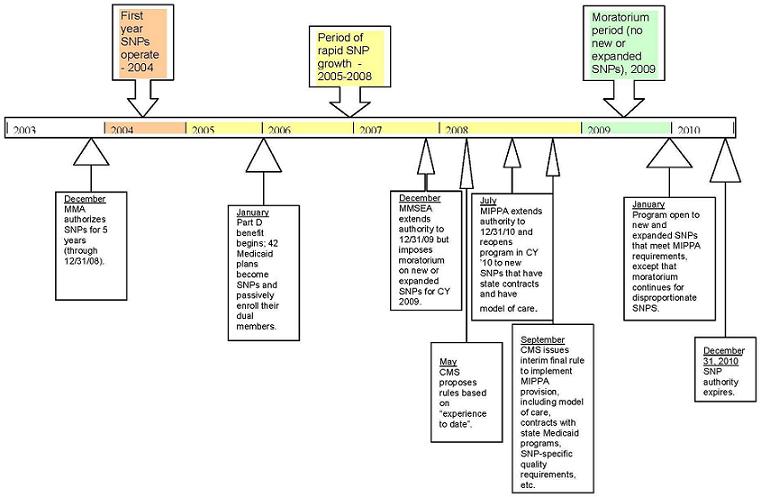 |
| SOURCE: Thomson Reuters, 2008. |
December 2003: MMA Authorizes the Creation of SNPs
Section 231 of the MMA of 2003 authorized Specialized Medicare Advantage plans for special needs individuals as a new type of Medicare managed care plan now known as SNPs. Unlike other Medicare Advantage (MA) plans, which must enroll all qualified Medicare beneficiaries, SNPs may restrict enrollment to one of three specialty groups of Medicare beneficiaries:
an MA eligible individual who-- (i) is institutionalized (as defined by the Secretary); (ii) is entitled to medical assistance under a State plan under title XIX; or (iii)meets such requirements as the Secretary may determine would benefit from enrollment in such a specialized MA plan described in subparagraph (A) for individuals with severe or disabling chronic conditions.3
The Secretary of Health and Human Services (HHS) subsequently defined category (i) in rules as those residing or expecting to continuously reside for 90 days or longer in a nursing home. Intermediate care facility for persons with mental retardation (ICF/MR), or an inpatient psychiatric facility. Category (ii) refers to beneficiaries who are dually eligible for Medicare and Medicaid, and category (iii) has focused on beneficiaries with particular diseases or chronic conditions, such as diabetes, hypertension, obesity, pulmonary disease and dementia.
For all three categories, the Secretary was given discretion to allow SNP designation not only for plans wishing to serve a group exclusively, but also for plans serving a disproportionate share of members in one of the categories. Disproportionate share was defined in rules as enrolling a greater proportion of special needs individuals than occur nationally in the Medicare population as defined by CMS.
As MA plans, SNPs must meet general MA program requirements, but beyond the general rules, CMS adopted very few rules pertaining specifically to SNPs, giving itself discretion to evaluate what might emerge in the market on a case-by-case basis. The original statutory and regulatory authority for the program is summarized at the top of Table 1.
2004-2007: Early Program Experience
The MMAs single page authorizing SNPs was easily eclipsed by the 105 pages creating the new prescription drug benefit, but it did not escape notice. Since the early days of state experimentation with integrated care models for dually eligible beneficiaries, state Medicaid and federal CMS officials have struggled to overcome barriers to integrated care.4 Soon after passage of the MMA, several observers noted the potential for SNPs to become mainstream vehicles for integrating Medicare and Medicaid services.5 CMS worked internally to support this direction, launching an Integrated Care Initiative in December 2005 to remove administrative barriers to implementing SNPs and to generate State awareness of the opportunity to better integrate care for individuals who are dually eligible for both Medicare and Medicaid.6 In March 2006, CMS published a guide to integrated care with the stated intent to convey the possibilities of an integrated program that works within existing or proposed regulations, is valuable to dual-eligible beneficiaries, meets State Medicaid Plan objectives, and is marketable for MA Organizations.7
The provision also was noticed by health plans, who embraced the SNP designation to an extent that surprised most observers. Table 2 shows the rapid growth in the program, from 11 SNPs in 2004 to 125 in 2005, and 276 in 2006. In 2006, the Medicare Payment Advisory Commission (MedPAC) took notice, observing that [t[heoretically, SNPs may improve care coordination for dual-eligibles and other special needs beneficiaries through unique benefit design and delivery systems. However, we are concerned that many SNPs are not designed to better coordinate care for special needs beneficiaries. SNPs, even dual-eligible SNPs, are not required to contract with states to provide Medicaid benefits, and many appear not to do so.8 By June 2007, MedPAC had commissioned a study on SNPs and noted its rising concern: Based on site visits and additional discussions with experts, we do not see how dual-eligible SNPs that do not integrate Medicaid could fulfill the opportunity to coordinate the two programs.9
Later in 2007, with support from the Commonwealth Fund, the Center for Medicare Advocacy held an invitational meeting with consumers, advocates, state officials, SNPs, federal officials and others to examine the SNP experience from the beneficiary perspective. Advocates had increased their focus on SNPs as the Medicare Part D drug benefit was implemented in 2006. To promote continuity for dually eligible beneficiaries who were already enrolled in state Medicaid managed care plans, 42 Medicaid plans in 13 states became SNPs, primarily so they could offer Part D benefits to their existing members, who would be transitioning from Medicaid drug benefits to Part D benefits.10 To make the transition as seamless as possible for beneficiaries, CMS authorized a one-time only passive enrollment of existing Medicaid managed care members into a set of newly approved companion SNP plans. The result was uneven. In Pennsylvania, coordination of benefits problems led the Pennsylvania Health Law Project to file a class action suit against CMS and reached a settlement to stop passive enrollment in that state. In other states, including Massachusetts, Minnesota and Wisconsin, where passive enrollment was employed to transition dual eligibility demonstration plans to SNP status, the process worked more smoothly, but the coordination challenges that emerged elsewhere served to point out the dearth of federal SNP requirements, particularly in relationship to state Medicaid programs. The Center for Medicare Advocacy recommended in October 2007 that the Federal Government require all SNPs to provide coordination of care and benefits with those offered by state Medicaid programs.11
December 2007: MMSEA Extension and Moratorium
Section 108 of the MMSEA of 2007 offered both good news and bad news to SNP supporters. On the one hand, federal authority for SNPs, which had originally been set to expire on December 31, 2008, was extended for an additional year to December 31, 2009. On the other hand, a moratorium was placed on CMSs authority to approve any new plan or plan expansion that was not already approved to enroll members on January 1, 2008.
CMS offered guidance to plans in a January 2008 memorandum: MA Organizations may continue to offer existing CMS-approved SNPs through December 31, 2009. CMS will monitor and provide technical assistance to MAs with SNPs in accordance with existing contracts, but will not approve any reconfiguration of SNP type, SNP subset, or SNP service area.12 As a practical matter, CMS had already approved several new and expanded plans for operation in 2008, and since those approvals had been made before the MMSEAs enactment in December 2007, they were already approved to enroll members on January 1, 2008. As Table 2 illustrates, 2008 saw continued growth of the program to 770 SNPs, up from 447 in 2007. However, as directed by the moratorium, CMS has not accepted any applications in 2008 for new or expanded plans to operate in 2009. Existing plans may continue to operate and continue to accept new members, but they will not be allowed to serve new areas in 2009. Table 1 summarizes these changes to the program.
May 2008: CMS Publishes Proposed Rule
On May 16, 2008, noting that we have gained more experience with the MA program, CMS published a proposed rule that included changes to the SNP program.13 As outlined in Table 1, the rule proposed:
-
Replacing the disproportionate share rule with a 90% rule, in which 90% of enrollees would need to be special needs individuals targeted by the SNP. CMS noted in the proposed rule that disproportionate SNPs had proliferated, probably in part because SNPs are allowed to market year round, whereas regular MA plans can only market during the annual open enrollment period.
-
SNPs would need to employ a CMS-approved process for verifying members special needs status. In the case of dual eligibility SNPs, this would have included getting verification from the state.
-
More generally, dual-eligible SNPs would need a documented relationship with the state Medicaid agency that would include eligibility verification, Medicaid provider information, and Medicaid benefits information. This provision would have taken effect three years after effective date of rule, placing the effective date in late 2010.
-
SNPs would need to have a model of care plan specific to the needs of their members. This would have included care coordination, appropriate network, appropriate care protocols, care for frail and disabled members, and care at end of life.
July and September 2008: MIPPA Supercedes Aspects of Proposed Rule; CMS Issues Interim Final Rule
In July with CMSs proposed rule out for comment, Congress acted again, this time as part of the MIPPA of 2008, an urgent piece of legislation needed to stave off a Medicare rate cut for physicians. Section 164 extended federal SNP authority one more year, to December 31, 2010, and placed several new provisions in the statute, summarized in Table 1. Because MIPPA required certain provisions to be in place no later than November 15, 2008, CMS issued an Interim Final Rule with immediate effect on September 18, 2008, and has noted that it will finalize certain aspects of the May 16 Proposed Rule at a later date.
Major MIPPA provisions include:
-
The previous moratorium was turned into a one-year freeze for most plans. As previously prohibited under MMSEA, there will be no new or expanded SNPs in contract year 2009, but CMS will resume accepting applications in 2009 for contract year 2010. The exception is disproportionate share plans, which continue under a moratorium through 2010.
-
Beginning in 2010, new dual-eligible SNP applicants and dual SNPs seeking to expand their service areas must have a contract with the state Medicaid agency to provide or arrange for benefits to be provided under Medicaid. Existing dual SNPs that do not have a contract with the state Medicaid agency may continue to operate but may not expand their service areas. States are not required to enter into contracts with dual SNPs. CMS has not yet issued guidance on this provision, but has adopted the following language in its September Interim Final Rule:
Minimum contract requirements. At a minimum, the contract must document-- (1) The MA organizations responsibility, including financial obligations, to provide or arrange for Medicaid benefits. (2) The category(ies) of eligibility for dual-eligible beneficiaries to be enrolled under the SNP, as described under the Statute at sections 1902(a), 1902(f), 1902(p), and 1905. (3) The Medicaid benefits covered under the SNP. (4) The cost-sharing protections covered under the SNP. (5) The identification and sharing of information on Medicaid provider participation. (6) The verification of enrollees eligibility for both Medicare and Medicaid. (7) The service area covered by the SNP. (8) The contract period for the SNP.
Clearly, these provisions allow for different levels of relationship between SNPs and states, ranging from agreements to coordinate with one another to arrangements in which a state Medicaid program contracts with the SNP to fully integrate Medicaid benefits through the MA plan.
-
Also effective for contract year 2010, all SNPs are required to have a model of care that includes appropriate provider networks for the target population, including specialists, and a number of care management components, including initial assessment and annual reassessment of members, a comprehensive plan of care, and an interdisciplinary team. CMS had already moved to specify a model of care in its May proposed rule, and it has interpreted MIPPA as adding additional components, which have been incorporated in the September Interim Final Rule. CMS has offered the following guidance.14
The [2009] Call Letter guidance substantively fleshed out the SNP MOC architecture by describing eight components designed to support service delivery for special needs individuals. These components included: (1) Goals and objectives pertinent to the plans targeted special needs beneficiaries. (2) Comprehensive risk assessment using a risk assessment tool. (3) Specialized provider network. (4) Care coordination. (5) Service delivery system including protocols and out-of-network specialists. (6) Communication and accountability system. (7) SNP training for network providers. (8) Performance measurement and improvement activities.
MIPPA added new specific statutory requirements pertaining to a SNP MOC. Beginning January 1, 2010, all SNPs must not only have an evidence-based care model with specialized providers, but must also have care management services that add the following components: (1) A comprehensive initial health risk assessment and annual reassessment of the physical, psychosocial, and functional needs of the special needs individual. (2) A care plan for each beneficiary that addresses goals and objectives, services and benefits provided, and measurable outcomes. (3) An interdisciplinary team used in the care management of each beneficiary.
CMS goes on to offer possible ways that a SNP might develop and implement its plan of care, including relying on its Medical Director and staff to conduct research regarding on evidence pertaining to the target population, using the evidence referenced by the Agency for Healthcare Research and Quality on its website, using protocols developed by specialty societies, etc. Whatever approach is used, SNP management is accountable for being able to articulate the model of care and measuring its implementation.
Regarding interdisciplinary teams, CMS has signaled flexibility here as well, both in terms of composition and uniformity. SNPs may have a standard team, or may develop teams that vary according to their members needs.
-
MIPPA also expanded quality improvement (QI) program requirements for contract year 2010, adding SNP-specific provisions to the general MA QI requirements. In general, SNPs will be required to focus their QI efforts on measuring the existence and impact of specified model of care components. CMS has indicated its intent to require a three-tiered program:15
The first tier consists of the mandatory collection and reporting of data using 13 HEDIS measures and three structure and process measures CMS is collaborating with the National Committee on Quality Assurance (NCQA) on a three-year initiative to refine SNP reporting measures and make them relevant to special needs individuals.
The second tier reflects the QI requirements established in the January 28, 2005 final rule implementing changes to Part C made in the MMA of 2003 MIPPA elaborated on the QI program required under MMA by directing SNPs to collect, analyze, and report data measuring health outcomes and quality indices pertaining to special needs individuals at the plan level as well as measuring the effectiveness of care management and their model of care. SNPs can meet both directives by making the collection and analysis of health outcomes and quality indices pertaining to special needs individuals the focus of their QI projects and chronic care improvement program
CMS is developing the third tier of the QI program. This tier involves CMS monitoring of care management implementation through the collection, analysis, and reporting to CMS of selected data that measure the effectiveness of SNP models of care Additional guidance regarding the development of monitoring measures will be forthcoming.
2009 and Beyond: To Be Determined
The flurry of Congressional and CMS action in the pay year created much confusion, but now that the dust has settled, the current status of the SNP program is reasonably clear. Under current law, CMS may resume adding new and expanded SNPs as of January 1, 2010, but SNPs will also need to meet several new requirements on that date, with the model of care perhaps the most significant. Entering into contracts with states is also a significant new requirement for dual-eligible SNPs, though existing plans that are not able or willing to enter into such agreements have the option of remaining within their existing service areas for 2010.
Most observers believe that Congress will extend authority for the SNP program beyond its current authorization of December 31, 2010. Perhaps more significant is the extent to which program rates will be scaled back as part of a larger effort to bring MA costs per beneficiary closer to parity with traditional Medicare fee-for-service costs. When Medicare managed care rates were last reduced in the Balanced Budget Act (BBA) of 1997, a major market disruption occurred as several plans withdrew from the Medicare program.
Much has been learned since 1997 regarding how to improve care for dually eligible beneficiaries, and several states are interested potentially in partnering with SNPs to apply the lessons learned from the early adopter states. The BBA unleashed a strong backlash among Medicare beneficiaries who were displaced when their health plans withdrew from the Medicare program, calling into question the ability to build stable dual eligibility programs on a base of Medicare plans. Some states will conclude that the benefits outweigh the risks and will build new partnerships with SNPs, while other states wait to see what the next set of federal changes brings.
| TABLE 1. Federal SNP Authority, 2003 - 2008 | ||
| Federal Authority | Summary of Major Statutory Provisions | Summary of Major Rule Provisions |
| Statute: Section 231 of the Medicare Prescription Drug, Improvement, and Modernization Act (MMA) of 2003 Public Law No: 108-173, effective 12/8/2003 Rules: Revisions to 42 CFR Part 422, effective 3/22/05 (70 FR 4588-4741) | SNPs were authorized through 12/31/08, allowing Medicare Plans to restrict enrollment to the following special needs group:
The Secretary of HHS was given authority to recognize as SNPs plans that serve these groups exclusively or disproportionately. | Institutionalized was defined as continuously residing or expected to continuously reside for 90 days or longer in a skilled nursing facility, nursing facility, ICF/MR, or an inpatient psychiatric facility. Disproportionate share SNP was defined as one that enrolls a greater proportion of special needs individuals than occur nationally in the Medicare population, as defined by CMS. |
| Statute: Section 108 of Medicare, Medicaid, and SCHIP Extension Act (MMSEA) of 2007 Public Law No: 110-173, effective 12/29/2007 | Authorization was extended through 12/31/09. A moratorium was imposed on the approval of new SNPs or the expansion of existing SNPs beyond what had already been approved for offer on 1/1/08. Since applications for the 2008 contract year were approved earlier 2007, the effect of the moratorium was to freeze applications for new or expanded SNPs for contract year 2009. Existing plans were allowed to continue operating and taking new members in their existing service areas. | No new rules required. |
| On May 16, 2008, CMS published a discretionary Proposed Rule to amend 42 CFR Part 422, based on experience gained to date (73 FR 28556-28604) Some of the proposed provisions have been superceded by MIPPA (below). Others will be finalized by CMS | Disproportionate share definition would be replaced by a 90% rule (90% of members enrolled must be special needs individuals). Superceded by MIPPA moratorium on disproportionate share plans. See MIPPA below. SNPs would need to employ a process for verifying members special needs status. SNPs would need to have a model of care plan, specifying how they will meet the needs of their members. This would have included care coordination, appropriate network, appropriate care protocols, care for frail and disabled members, and care at end of life. May supplement MIPPA model of care provisions. Dual-eligible SNPs would need a documented relationship with the state Medicaid agency to include eligibility verification, Medicaid provider information, Medicaid benefits information. (Would have taken effect three years after effective date of rule.) Superceded by MIPPA state Medicaid contract provision. | |
| Statute: Section 164 of the Medicare Improvements for Patients and Providers Act (MIPPA) of 2008 Public Law No: 110-275, effective 7/15/2008 Rules: Revisions to 42 CFR Part 422, effective 9/18/2008 (73 FR 54226-54254) Because Congress directed that certain provisions of MIPPA take effect no later than November 15, 2008, CMS issued an Interim Final Rule to implement MIPPA, and has stated its intention to finalize its May 16 Proposed Rule at a later date. | Authorization is extended to 12/31/10. The freeze on new and expanded SNPs is lifted for contract year 2010, except that no new disproportionate SNPs may be approved. By 1/1/2010, new or expanding dual eligibility SNPs must have contracts with states to either: provide Medicaid benefits or coordinate them. (Existing dual eligibility SNPs may continue to operate without state contracts but may not expand to new service areas. States are not required to enter into contracts.) By 1/1/2010, all SNPs must have an evidence-based model of care that includes initial assessment, annual reassessment, a plan of care, and an interdisciplinary team. QI provisions are expanded to require that SNPs focus their QI efforts on monitoring the performance of their models of care. | State contract is defined as a formal written agreement between an MA organization and state Medicaid agency documenting each entitys roles and responsibilities with regard to dually eligible individuals. Minimum state contract requirements include: the SNPs obligations, including financial, to provide or arrange for Medicaid benefits; the categories of dually eligible beneficiaries to be enrolled; the Medicaid benefits covered under the SNP; the cost-sharing protections covered under the SNP; identification and sharing of information on Medicaid provider participation; eligibility verification; service area and contract period. Model of care must be an evidence-based approach that includes appropriate network capacity for the target population and must include that following care management provisions: a comprehensive initial assessment and annual reassessments of physical, psychosocial and functional needs; a comprehensive individualized plan of care developed and monitored by an interdisciplinary team. In addition to meeting general MA quality requirements, SNPs must measure outcomes and indices pertaining to their target population, and must focus QI efforts on measuring and improving their models of care. Self monitoring and CMS monitoring are expected to focus on effective implementation of all aspects of the care model. |
| TABLE 2. Special Needs Plans Over Time | ||
| Contract Year | Number of SNPs* | Notable |
| 2004 | 11 | First year of operation. |
| 2005 | 125 | |
| 2006 | 276 | Includes one-time only roll-over allowed for 42 Medicaid plans that acquired SNP status to continue providing drug benefits as members transitioned from Medicaid drug benefit to Medicare Part D. |
| 2007 | 477 | |
| 2008 | 770 | |
| 2009 | No more than 770 due to one-year freeze. | Moratorium in place; no new or expanded SNPs approved. |
| 2010 | May increase as new plans are again allowed, but may decrease as some plans drop out rather than meet new requirements. | |
| 2011 | Currently not authorized. | |
| * Source for 2004-2006 figures: Milligan, Charles J., Jr. and Cynthia H. Woodcock. 2008. Medicare Advantage Special Needs Plans for Dual Eligibles: A Primer. (The Commonwealth Fund, 2008.) Source for 2007-2008: CMS. September 2007. Special Needs Plan Comprehensive Report. Available at http://www.cms.hhs.gov/MCRAdvPartDEnrolData/SNP/list.asp. | ||
Notes
-
Section 231, MMA of 2003, PL 108-173, 12/8/03.
-
Section 108, MMSEA of 2007, PL 110-173, 12/29/07. Section 164, MIPPA of 2008, PL 110-275, 7/15/08.
-
Section 231(b)(6)(B).
-
For summary discussion of early barriers, see: General Accounting Office, Medicare and Medicaid: Implementing State Demonstrations for Dual Eligibles Has Proven Challenging, GAO/HEHS-00-94, Report to U.S. Senate, Special Committee on Aging, August 2000.
-
See, for example: Peters, Christie Provost. 2005. Medicare Advantage SNPs: A New Opportunity for Integrated Care? Issue Brief #808. National Health Policy Forum. November 11, 2005.
Saucier, Paul, Brian Burwell and Kerstin Gerst. 2005. The Past, Present and Future of Managed Long-Term Care. Prepared by Medstat and the University of Southern Maine for the U.S. Department of Health and Human Services, Office of the Assistant Secretary for Planning and Evaluation. http://aspe.hhs.gov/daltcp/reports/mltc.htm.
Medicare Advantage Special Needs Plans: New Opportunities for State Medicaid Programs. The Piper Report, September 24, 2005. http://www.piperreport.com/archives/2005/09/medicare_advant.html Accessed 9/24/08.
-
Integrated Care Initiative, Overview. http://www.cms.hhs.gov/IntegratedCareint/ Accessed 9/24/08.
-
CMS. State Guide to Integrated Medicaid and Medicare Models. (Draft) March 1, 2006.
-
MedPAC. June 2006. Report to Congress: Increasing the Value of Medicare, Chapter 9.
-
MedPAC. June 2007. Report to Congress: Promoting Greater Efficiency in Medicare, Chapter 3.
-
Recommendations of the Center for Medicare Advocacy. October 2007. http://www.medicareadvocacy.org/SNP%20Conference/Recommendations.htm Access 9/28/08.
-
CMS. January 24, 2008. Memorandum from David A. Lewis, Director, Medicare Advantage Group, to MA Plans regarding Moratorium on Special Needs Plans (SNP).
-
73 FR 28556-28604.
-
Excerpted from: CMS. September 15, 2008. Memorandum from Abby L. Block, Director, Center for Drug and Health Plan Choice to MA plans and other contractors regarding Guidance for Regulations in CMS 4131-F and CMS 4138-IFC.
Issue Briefs on Special Needs Plans
A total of three Issue Briefs are available from the Office of Disability, Aging and Long-Term Care on this subject:
-
Federal Authority for Medicare Special Needs Plans and Their Relationship to State Medicaid Programs, http://aspe.hhs.gov/daltcp/reports/2009/leghist.htm (Posted April 2009)
-
State Purchasing Strategies Drive State Contracts with Medicare Special Needs Plans, http://aspe.hhs.gov/daltcp/reports/2009/stpur.htm (Posted June 2010)
- Medicaid Contracts with Medicare Special Needs Plans Reflect Diverse State Approaches to Dually Eligible Beneficiaries, http://aspe.hhs.gov/daltcp/reports/2009/SNPdual.htm (Posted June 2010)
To obtain a printed copy of this report, send the full report title and your mailing information to:
U.S. Department of Health and Human ServicesOffice of Disability, Aging and Long-Term Care PolicyRoom 424E, H.H. Humphrey Building200 Independence Avenue, S.W.Washington, D.C. 20201FAX: 202-401-7733Email: webmaster.DALTCP@hhs.gov
RETURN TO:
Office of Disability, Aging and Long-Term Care Policy (DALTCP) Home [http://aspe.hhs.gov/_/office_specific/daltcp.cfm]Assistant Secretary for Planning and Evaluation (ASPE) Home [http://aspe.hhs.gov]U.S. Department of Health and Human Services Home [http://www.hhs.gov]
